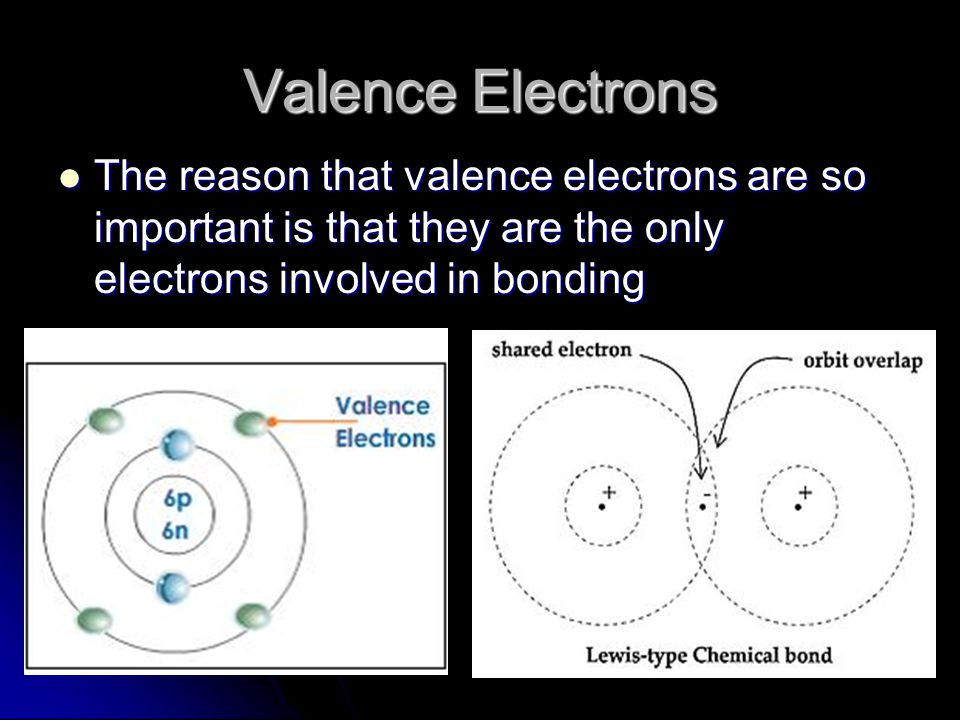
The bonding between the two atoms is due to the interaction of those. The bonding between the two atoms is due to the interaction of those.

Valence electrons are important as they indicate an elements bonding behavior stability and reactivity.
Why are valence electrons so important. Why Are Valence Electrons Important. Valence electrons are the electrons in the outermost energy level of an atom in the energy level that is farthest away from the nucleus. When chemists study chemical reactions they study the transfer or sharing of electrons.
Why are valence electrons important. Are the outer electrons and therefore they are the first ones to interact when two atoms come near each other. The bonding between the two atoms is due to the interaction of those.
Whether they share them or give-or-take them it is almost exclusively the electrical forces of the valence electrons that holds the atoms together. If the valence electrons of elements are really close or really far to 8 like 1 or 7 those elements tend to be very reactive and dont generally have a lot of oxidation states. The alkali metals group 1 elements each have 1 valence electrons so they tend to be very reactive and readily lose that electron.
The halogens group 7 or 17 elements each have 7 valence electrons and will react with almost. Valence electrons are important as they indicate an elements bonding behavior stability and reactivity. Note that the number of valence electrons.
Note that the number of valence electrons. Why are valence electrons important. Are the outer electrons and therefore they are the first ones to interact when two atoms come near each other.
The bonding between the two atoms is due to the interaction of those. Whether they share them or give-or-take them it is almost exclusively the electrical forces of the valence electrons that holds the atoms together. Directory of Chem Help ASAP videos.
Valance electrons that reside in the outermost shell of an atom in the highest energy level. They are important to atoms because the fewer valence electrons that the atom holds it becomes less stable. Answer A is true but not the most important reason.
Valence electrons tell us how compounds will bond by assuming all atoms would like to have 8 valence electrons. Valence electrons tell us how. Why are valence electrons important See answers 2 Ask for details.
Follow Report Middle School. Why are valence electrons important aniyawalker2003 Asked 12182019. Why are valence electrons important.
Make friends and ask your study question. Why are valence electrons important. Atoms Molecules and Ions.
An Active Learning Approach 6th. The Quantum Model of the Atom Section 5. You must be signed in to discuss.
Top Chemistry 102 Educators. Understanding valence electrons is key to understanding chemical bonding. Delocalized valence electrons produce metallic bonds bonds between atoms of metals which give metals unique properties such as conductivity and ductility draw metal into wires.
Valence electrons are the electrons that are shared between atoms which are covalently bonded. The electrons that occupy the outer most shell of an atom are called valence electrons. They are important because they determine how an atom will react.
By writing an electron configuration Youll be able to see how many electrons occupy the highest energy level. The number of valence electrons the number of electrons in the outer orbital lets the atom combine with other elements that want more valence electrons. I also believe that the number of valence electrons of an element determine what group on the periodic table of elements it is in.
Valence electrons are important as they are the ones which take part in chemical reactions. They have more energy as compared to the other electrons of. The valence electrons of an atom are responsible for and take part in chemical changes.
The valence electrons in an atom determine the mode of chemical combination ie whether the atom in combining with other atoms can form ionic or covalent bonds is determined by the number of valence electrons present in the atom.