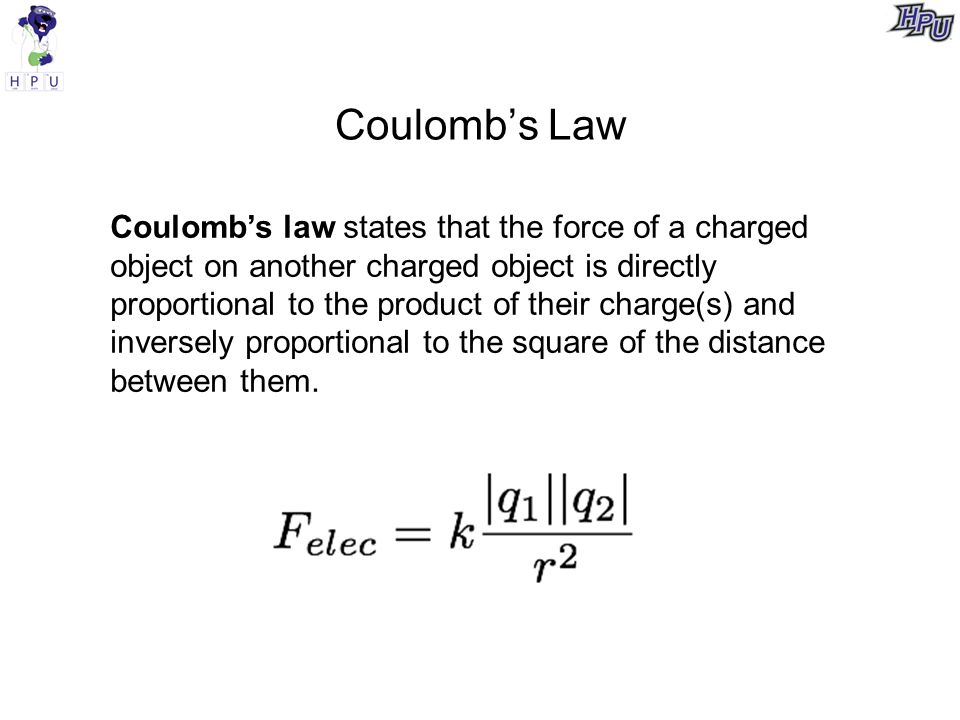
Coulombs Law Definition. But scientists were clueless about various factors affecting strength of charge.
Coulombs Law is one of the basic ideas of electricity in physics.
What is coulombs law simplified. Coulombs law is a function developed in the 1780s by physicist Charles-Augustin de Coulomb. It explains how strong the force will be between two electrostatic charges. Electrostatic means electric charges without any motion.
Coulombs Law Definition. Colombs law states that the magnitude of the electrostatic force of attraction or repulsion between two electrically charged bodies is directly proportional to the product of the charge of the charged bodies and inversely proportional to the square of the distance between the center of the charged bodies. What is Coulombs Law.
According to Coulombs law the force of attraction or repulsion between two charged bodies is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. It acts along the line joining the two charges considered to be point charges. The second law of electrostatics also known as Coulombs law states the force exerted between two point charges is.
Directly proportional to the multiplication of the magnitude of both charges. Inversely proportional to the square to the square of the distance between them and. In physics Coulombs law describes the forces acting between electric point charges.
The law was first given by Charles-Augustin de CoulombIt is an inverse-square law for two electric charges very similar to Newtons gravitational law for two masses. An important difference between Newtons and Coulombs law is that masses always attract each other whereas charges may repel or attract. Scientists in 18th century knew that particle that is electrically charged would exert certain force on another charged particle.
But scientists were clueless about various factors affecting strength of charge. This problem was finally solved by Charles Coulomb when he proposed the famous Coulombs Law. The force between two point charges is directly proportional to the magnitude of each charge q 1 q 2inversely proportional to square of the separation between their centers rdirected along the separation vector connecting their centers rThis relationship is known as Coulombs Law.
Charles-Augustin Coulomb 17361806 France. Limitations of Coulombs law. Coulombs law is applicable to point charges only and is not applicable to extended bodies.
It can be applied to extended bodies by assuming them to be point charges but the distance between them should be sufficiently large The law holds good only for stationary charges. Charles-Augustin de Coulomb discovered the Laws of Electrostatics in 1785 known as Coulombs Law. Until 1784 no one knew about the unit of the electric charge then the Coulomb introduced these laws after multiple experiments on force between two masses based on the Inverse Square Law.
Coulombs laws of electrostatic can be stated as follow. Coulombs Law is one of the basic ideas of electricity in physics. The law looks at the forces created between two charged objects.
As distance increases the forces and electric fields decrease. This simple idea was converted into a relatively simple formula. Coulombs Laws Of Electrostatics.
Like charges repel each other and unlike charges attract each other. In other words bodies having same charges repel each other and bodies oppositely charged attract each other. The first law tells only about the nature of force ie repulsive or attractive.
General Physics the principle that the force of attraction or repulsion between two point electric charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. A similar law holds for particles with mass. Coulombs law mathematical description of the electric force between charged objects.
Formulated by the 18th-century French physicist Charles-Augustin de Coulomb it is analogous to Isaac Newtons law of gravity. Learn more about Coulombs law in this article. The magnitude of the force is given by Coulombs Law.
12 2 12 12 2 3 qq F KKq x F Since the force is non-zero and repulsive the charges will accelerate in the directions specified by the forces. Can we add a third charge to counteract.
