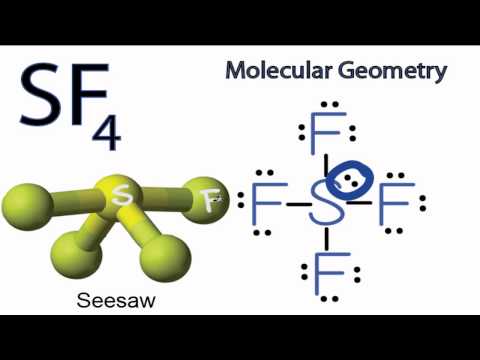
Therefore it is polar. Therefore this molecule is polar.
Sinc SF4 has symmetry its shape is tetrahedral it is a non polar molecule with polar bonds COF2 carbonyl fluoride is a slightly polar molecule.
Sf4 polar or nonpolar. The sulfur tetrafluoride SF4 is a polar molecule because in SF4 lone pair is one. Therefore it is polar. If the lone pair is odd then it will be polar but if the lone pair is even it will be nonpolar.
With that its also essential to know about the BF3 NH4 and SO3 polarity. The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and if necessary check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom the molecule is polar.
If there is an even number of lone pairs you must check the VSEPR structure to decide. In your example of SF_4 the Lewis Structure would look. Answer SF4 sulfur tetrafluoride is Polar What is polar and non-polar.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an.
Is SF4 nonpolar or polar. The Correct Answer is. Polar is correct for is SF4 nonpolar or polar.
Learn to determine if SF4 Sulfur tetrafluoride is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewi. The geometry of Sf4 will be asymmetric electron region distribution around the central atom S. Therefore this molecule is polar.
Source of attached structure images. 2- SF4 Lewis and 3-D Structure- Dr. Here is the answer for the question is SF4 nonpolar or polar.
Youll find the correct answer below is SF4 nonpolar or polar The Correct Answer is polar Reason Explained polar is correct for is SF4 nonpolar or polar Answerout. CF4 is a nonpolar molecule. Although all C-F bonds are polar because carbon and fluorine differ in their electronegativity the overall CF4 molecule is non-polar.
This is because of the symmetrical arrangement of all fluorine atoms around the central carbon atom. Therefore each individual C-F bond dipole cancels out each other resulting in the net-zero dipole moment of the entire molecule. Sinc SF4 has symmetry its shape is tetrahedral it is a non polar molecule with polar bonds COF2 carbonyl fluoride is a slightly polar molecule.
All the bonds are polar. Also question is is sf4 a polar or nonpolar molecule. The other explanation goes like this.
Two S-F bonds are opposite from each other in complete 180 degrees. But the other two S-F bonds are pointing down and that is why their bond dipoles do not cancel. So SF4 molecule is polar.
If the charge distribution is symmetric it is non-polar. Answer SiF4 Silicon tetrafluoride is Nonpolar What is polar and non-polar. The Phosphorus Pentafluoride PF5 is a nonpolar molecule because it is considered vertical and horizontal planes.
To achieve a neural conformation the electrons pull balance themselves. To achieve a neural conformation the electrons pull balance themselves. SiF4 is a nonpolar molecule because the fluorines are arranged around the central silicon atom in a tetrahedral molecule with all of the.
Here SF4 bond angles are around 102 degrees in the equatorial plane and around 173 degrees between the axial and equatorial positions. Are sulfur tetrafluoride SFA and nitrogen trifluoride NF3 polar or nonpolar. Select all that apply SFA is polar.
Quiz your students on Lewis Structure For ArF4 Molecular Geometry Hybridization Bond Angle Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching. Ill tell you the polar or nonpolar list below. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
List molecules polar and non polar. Hydrogen sulfide is non-polar on account of its non polar HS bonds. N With 2P-orbitals there are overlapped four of the hybrid orbitals.
2 fluorine atoms are present either side of the molecule. If there are no polar bonds the molecule is nonpolar.
