Lewis Structure For Pf6 Mrdchemgwiki Solution Chemistry lewis structure for pf6 new 4 0 dp chemistry. HCl 2 electron groups sp hybridization EX.
A step-by-step explanation of how to draw the NH3Cl Lewis Dot StructureFor the NH3Cl structure use the periodic table to find the total number of valence.
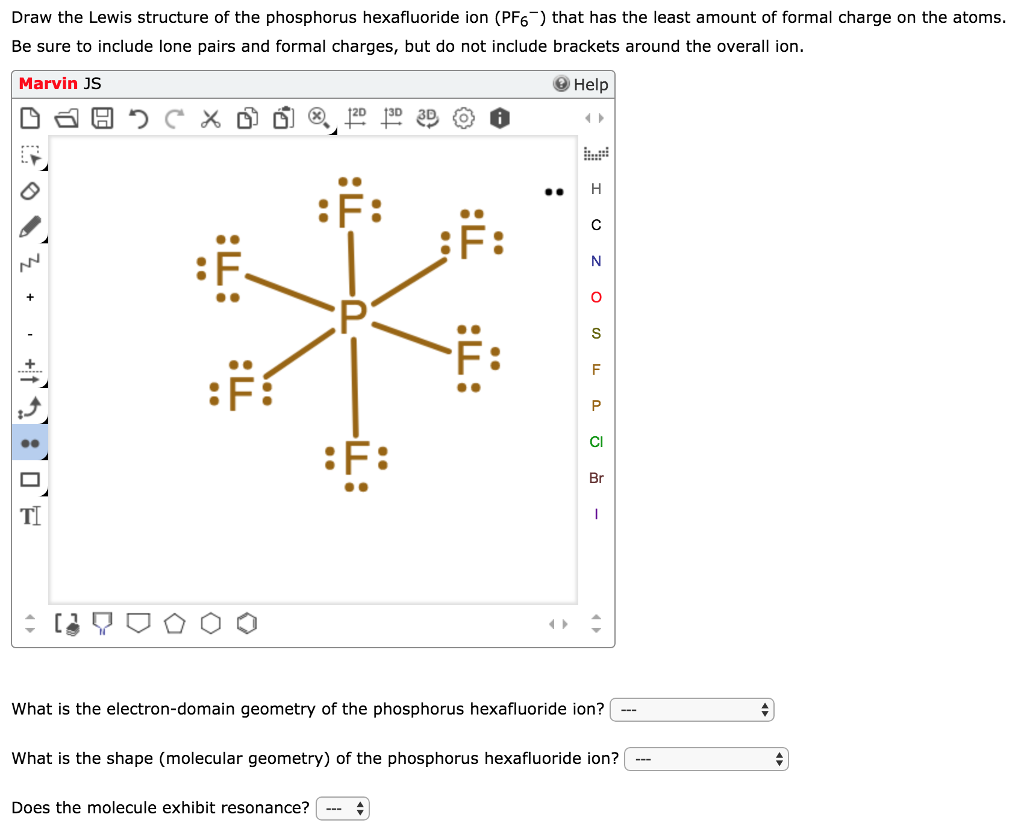
Pf6 lewis structure. There are a total of 48 valence electrons in PF 6-. Phosporous P is the least electronegative and goes at the center of the Lewis structure. Phosporous P is below Period Two on the periodic table and can hold more than 8 valence electrons.
Be sure to put brackets and a negative sign around the PF 6- Lewis structure to show that it is an ion. A video explanation of how to draw the Lewis Dot Structure for the Hexafluorophosphate Ion along with information about the compound including Formal Charge. This is the PF6- Lewis structure.
Phosphorus has 5 valence electrons. We have 6 Fluorines and we need to add in this valence electron up here for a total of 48 valence electrons. Phosphorus is the least electronegative.
Well put that at the center and then well put the 6 Fluorines around the outside. PF6- has a total of 48 electrons and there are 6 bonds to the 6 fluorine atoms. Therefore P has 6 electron pairs.
The polyatomic ion has octahedral molecular. In general the typical pattern for basicsimple molecules is. Diatomic molecule No hybridization at all.
HCl 2 electron groups sp hybridization EX. CO_2 3 electron groups sp2 hybridization EX. BF_3 4 electron groups sp3 hybridization EX.
CH_4 5 electron groups sp3d hybridization EX. PF_5 6 electron groups sp3d2 hybridization EX. SF_6 You can.
Owing to the Lewis acidity of the Li ions LiPF 6 also catalyses the tetrahydropyranylation of tertiary alcohols. In lithium-ion batteries LiPF 6 reacts with Li 2 CO 3 which may be catalysed by small amounts of HF. LiPF 6 Li 2 CO 3 POF 3 CO 2 3 LiF Application.
Hexafluorophosphate F6P- CID 9886 - structure chemical names physical and chemical properties classification patents literature biological activities. Lewis Structure For Pf6 Mrdchemgwiki Solution Chemistry lewis structure for pf6 new 4 0 dp chemistry. A step-by-step explanation of how to draw the PCl6- Lewis Dot Structure Because Phosphorous is below Period row Two on the periodic table it can hold more.
Valence electrons on central atom. 6 F each contribute 1 electron. Add one for the negative charge on P.
Divide by 2 to give electron pairs. Octahedral geometry for the six shape-determining electron pairs. A Draw The LEWIS STRUCTURE For The PF6 Ion.
B What Is The ELECTRON PAIR GEOMETRY Around The Central Atom. C What Is The Shape Of The PF. D Is The PF.
E What Is The Bond Angle Around The Central Atom In The PF Ion. Based On Your Answer What Do You Think The Hybridization Would Be On The Central Atom. To predict the shape of the molecules first draw out the Lewis structure of the molecule.
On the Lewis diagram identify the central atom. For this molecule PF 6-the central atom is Phosphorus P. To work out how many electrons are in the outer shell of the.
Draw a Lewis structure for each. Select the type of octet-rule exception. A PF6-expanded outer shell free radical-incomplete octet incomplete octet b AsF4-expanded outer shell free radical-incomplete octet incomplete octet c ClO3 expanded outer shell.
There are 4251 valence electrons 48 6 initial bonds leaving 36 more electrons to distribute. These go around the F atoms. So P as the central atom and 6 single bonded F atoms around it.
Trisulfide2- S3-2 CID 5460599 - structure chemical names physical and chemical properties classification patents literature biological activities safety. A step-by-step explanation of how to draw the NH3Cl Lewis Dot StructureFor the NH3Cl structure use the periodic table to find the total number of valence.
