Now the metalloid which is present in period from left to right three on the periodic table is silicon. Thus silicon is the correct answer.
Thus silicon is the correct answer.
Metalloid in period 3. What is the only metalloid in period 3. A metalloid is a chemical element that exhibits some properties of metals and some properties of nonmetals. One metalloid of period 3 is Silicon.
Among the choices the only two metalloids are Silicon and Germanium. Germanium is in period 4 while silicon is in period 3. Aluminum is a metal and Phosphorus is a non-metal.
The only metalloid in period 3 is silicon. HOPE IT WILL HELP YOU FRIEND 1. 10 points What is the only metalloid in period 3.
Ask for details. Follow Report by Saugatpandey2934 09042019 Log in to add a. Some allotropes of elements show more pronounced metal metalloid or non-metal behaviour than others.
2 He 4. Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. The period 3 elements are.
Sodium magnesium aluminium silicon phosphorus sulfur chlorine and argon. Trends in physical properties across period 3 from left to right. A solids to gases chlorine and argon are the gases b good conductors sodium magnesium aluminium to insulators.
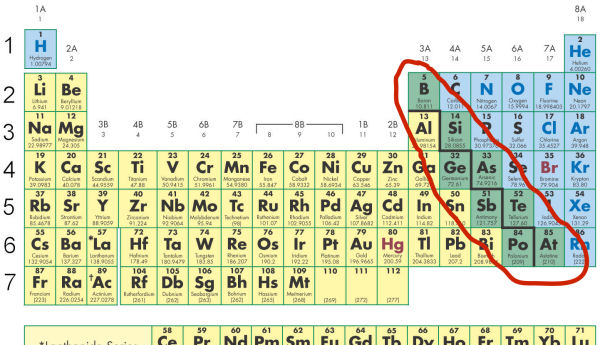
The series of these six elements is said to be metalloids. In the attached image six elements are present in the right side and in grey color are metalloids. Now the metalloid which is present in period from left to right three on the periodic table is silicon.
Thus silicon is the correct answer. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
Despite the lack of specificity the term remains in use in the literature of chemistry. What element is a metalloid in group 3. - 17415721 yoseline6149 yoseline6149 09042020 Chemistry High School 1.
What element is a metalloid in group 3. What group 1 element is a NON metal. 3what period 3 element has 8 valence electrons 1 See answer yoseline6149 is waiting for your help.
Add your answer and earn points. The graph shows how melting points and boiling points vary across period 3. There is a lot going on in this graph so it is often easier to divide it into three sections.
The table below gives a. 209 Number of ProtonsElectrons. 84 Number of Neutrons.
Pierre and Marie Curie.
