
Hydrogen H atoms always go on the outside of a Lewis structure. Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule.
NH4 Lewis Structure - How to Draw the Dot Structure for NH4 Ammonium Ion A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc.
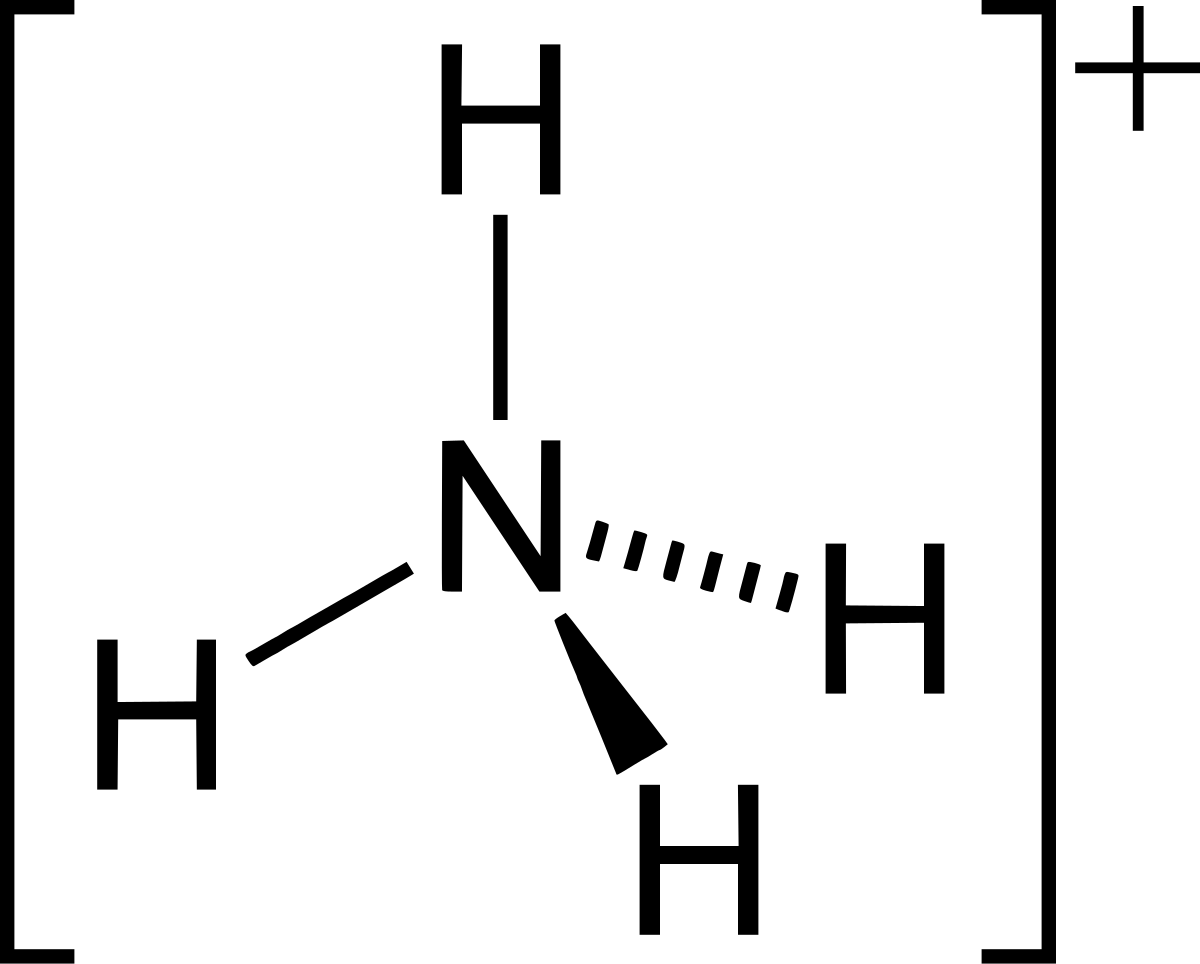
Lewis structure of nh4. What is the NH4 Lewis Structure. The structure will have a central N atom covalently bonded to 4 H atoms. The shape of this ion will be tetrahedral.
Steps of drawing lewis structure of NH 4 Find total number of electrons of the valance shells of hydrogen atoms and nitrogen atom Total electrons pairs as lone pairs and bonds Center atom selection Mark lone pairs on atoms Mark charges on atoms if there are charges on atoms. NH4 is known as an ammonium ion but NH4 would not make sense as it is not balanced. The lewis structure would be the Nitrogen atom in the center with 4 hydrogens surrounding it.
There would be no lone pairs the molecule would also be nonpolar. NH4 Lewis Structure - Ammonium Ion - YouTube. This chemistry video tutorial explains how to draw the lewis structure of the Ammonium Ion NH4My Website.
NH4 Lewis Structure - How to Draw the Dot Structure for NH4 Ammonium Ion A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc. How to Draw the Lewis Dot Structure for NH4NO3. Ammonium nitrate - YouTube.
Secret Deodorant Good for You Good for Them Good for Us AllStrengthNoSweat. For NH43PO4 we have an ionic compound and we need to take that into account when we draw the Lewis Structure. Well first draw the Ammonium ion NH4 and.
NH4 Lewis structure The Correct Answer is Remember that a plus sign means there is one LESS electron So its 541-18 electronsN will have a 4 Hs attached to it the single bonds should be enough for H and N forms an octet. A step-by-step explanation of how to draw the NH4OH Lewis Dot StructureFor NH4OH we have an ionic compound and we need to take that into account when we dra. Hello GuysIn this video we will learn about the structure of ammonium ion.
As it gives away one electron it attains a positive charge which is represented. The NH4 Lewis structure has a total of 8 valence electrons. Hydrogen H atoms always go on the outside of a Lewis structure.
Also note that you should put the NH4 Lewis structure in brackets with as 1 on the outside to show that it is an ion with a. Lewis and Geometrical Structure of NH4 As we know nitrogen has 5 valence electrons and requires 3 electrons to complete its octet. On the other hand hydrogen has 1 valence electron and requires 1 more electron to get stable.
So all four hydrogen atoms. Lewis Dot of the Ammonium Ion. 70 More Lewis Dot Structures.
Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. The exception of course being the hydrogens. They follow the duet rule 2 electrons.