Therefore when the salt is completely dissociated in an aqueous solution it forms NH4 and Cl- ions. Chemistry Organic Chemistry Acids and Bases Elements and Compounds.
Next about whether it is strong or not.
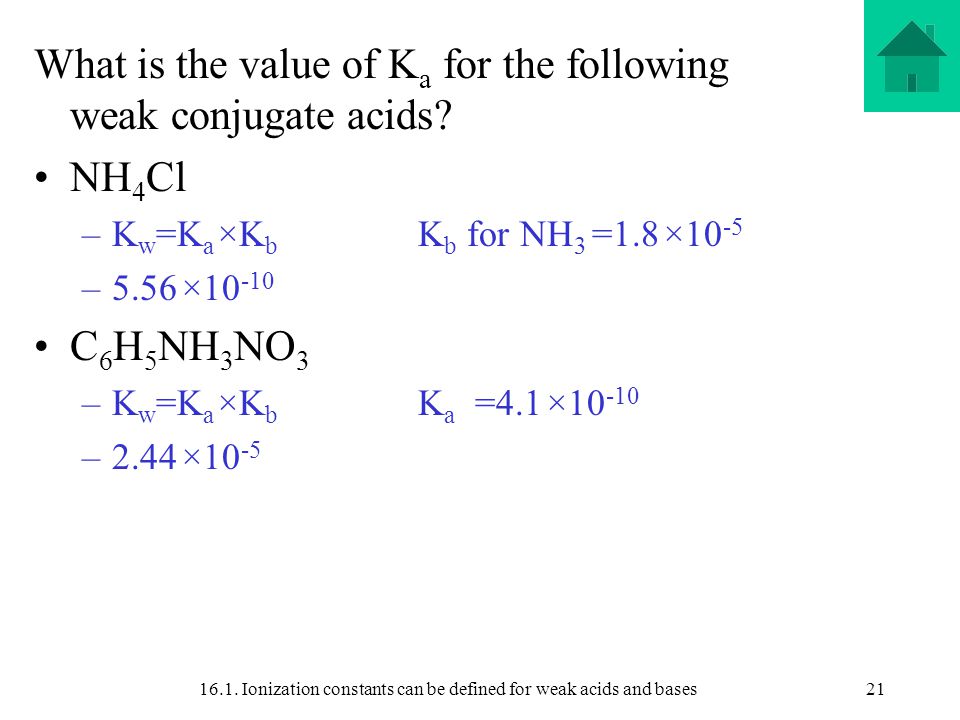
Is nh4cl a weak acid. Ammonium chloride NH4Cl will make acidic solution when dissolved in water due to hydrolysis of ammonium ion. Its aqueous solution is weakly acidic. Its acidity may be confirmed by Litmus test.
NH4Cl AMMONIUM CHLORIDE is acid What is an acid base neutral. Chemistry Organic Chemistry Acids and Bases Elements and Compounds. As mentioned in the other answer NH4Cl is an acidic salt formed by the neutralization of a strong acid HCl with a weak.
To tell if NH4Cl Ammonium chloride forms an acidic basic alkaline or neutral solution we can use these three simple rules along with the neutralization. NH4 aq H2O l H3O aq NH3 aq So ammonium ion is an acid as it donates H to form H3O. Next about whether it is strong or not.
The NH4Cl is an acidic salt. It dissociates into NH4- and Cl-. It is formed from reation of base ammonia and HCl acid which is one of the strong acid there for it is acidic salt.
As mentioned in the other answer NH4Cl is an acidic salt formed by the neutralization of a strong acid HCl with a weak base NH3. Therefore when the salt is completely dissociated in an aqueous solution it forms NH4 and Cl- ions. Is calcium carbonate a weak base.
While technically a weak acid hydrofluoric acid is extremely powerful and highly corrosive. Strong Acids Strong acids dissociate completely into their ions in water yielding one or more protons hydrogen cations per molecule. Ammonium carbonate is a very weakly acidic compound based on its pKa.
Consequently is NH4Cl an acid or base. As mentioned in the other answer NH4Cl is an acidic salt formed by the neutralization of a strong acid HCl with a weak base NH3. Therefore when the salt is completely dissociated in an aqueous solution it forms NH4 and Cl- ions.
Is NH4I acidic basic or neutral dissolved in water. If playback doesnt begin shortly try restarting your device. Ammonium chloride chemical formula NH4Cl is an acidic salt since it is a salt of a strong acid namely hydrochloric acid and a weak base namely ammonium hydroxide.
Was this answer helpful. Get the detailed answer. The conjugate acid of ammonia is NH4 a weak acid.
If a 02 MNH4Cl solution has a pH of 50 what is the Ka of NH4.
