
It is found in a wide variety of minerals but mainly it is found in marcasite and pyrite. The chemical formulas for iron nitrate and sodium phosphate are FeNO32 and Na3PO4.
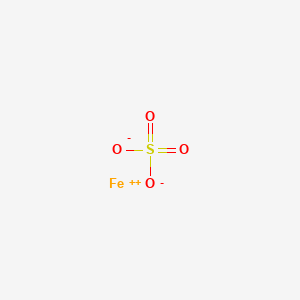
Iron2 sulfate anhydrous is a compound of iron and sulfate in which the ratio of iron2 to sulfate ions is 11.
Iron ii sulfate formula. The iron II sulfate chemical formula is FeSO 4. The molar mass is 15191 gmol. This salt is found in a broad range of hydrated salts with the formula FeSO 4xH 2 O while the most widely used is the heptahydrate iron II sulfate FeSO 47H 2 O with the molar mass of 27802 gmol.
The molecular or chemical formula of Iron II sulfate is FeSO 4. Iron II sulfate is a crystalline solid either greenish or yellow-brown. It is dangerous to the environment and quick measures should be taken to stop it.
It acts as a reducing agent. A compound of iron and sulfate in which the ratio of iron2 to sulfate ions is 11. Various hydrates occur naturally - most commonly the heptahydrate which loses water to form the tetrahydrate at 5 7C and the monohydrate at 65C.
IronII sulfate formula also known as Ferrous Sulfate formula or Green vitriol formula Is a compound of sulfate and iron in where the ratio of iron to sulfate ion is 11. Visit CoolGyan to learn more about the IronII sulfate formula structure properties. Iron2 sulfate anhydrous is a compound of iron and sulfate in which the ratio of iron2 to sulfate ions is 11.
Various hydrates occur naturally - most commonly the heptahydrate which loses water to form the tetrahydrate at 57 and the monohydrate at 65. It has a role as a reducing agent. It is a metal sulfate and an iron molecular entity.
Ammonium ferrous sulfate hexahydrate Ammonium ironII sulfate Mohrs salt Linear Formula. NH 4 2 FeSO 4 2 6H 2 O Molecular Weight. Iron II sulfate react with potassium permanganate and sulfuric acid 10FeSO 4 2KMnO 4 8H 2 SO 4 5Fe 2 SO 4 3 2MnSO 4 K 2 SO 4 8H 2 O Check the balance Iron II sulfate react with potassium permanganate and sulfuric acid to produce iron III sulfate manganese II sulfate potassium sulfate and water.
IronII sulfate heptahydrate 98. Find Sigma-Aldrich-310077 MSDS related peer-reviewed papers technical documents similar products more at Sigma-Aldrich. The chemical formulas for iron nitrate and sodium phosphate are FeNO32 and Na3PO4.
What Is The Formula Equation For Barium Chloride Sodium Hydroxide. Bariums ionic charge is 2 and chlorines is 1- meaning you need 2 chlorine ions for each barium ion. What Is The Balanced Equation For Sodium Oxide Combines With Water To Form Sodium Hydroxide.
For preparation of 10000 g of iron II sulfate heptahydrate а 3232 g of hydroxide or 2584 g of oxide and 9535 g of 37 sulfuric acid is required. Add acid to the flask then add small parts of iron compound with stirring until it will totally dissolve or if you use carbonate until carbon dioxide emission will stop. FeH 12 O 10 S.
CID 23925 Fe CID 1118 Sulfuric acid CID 962 Water Dates. 1 Structures Expand this section. 2 Names and Identifiers.
The formula unit for ironII sulfate is FeSO4. To calculate the formula mass multiply the subscript of each element times its atomic weight from the periodic table then add. Iron sulfate is a family of inorganic compound with the formula Fe2SO43H2On.
A variety of hydrates are known in fact are the most commonly encountered form of ferric sulfate. Solutions are used in dyeing as a mordant and as a coagulant for industrial wastes. It is also used in pickling baths for aluminum and steel.
10 2010-04-08 221126 UTC 2018-05-29 011417 UTC FDB014740 IronII sulfate FeSO4 IronII sulfate BrE. IronII sulphate or ferrous sulfate is the chemical compound with the formula FeSO4. Known since ancient times as copperas and as green vitriol the blue-green heptahydrate is the most common form of this material.
All iron sulfates dissolve in water to give the same aquo complex. Chemical Elements Periodic Table Compound Name Formula Search Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar Mass Calculator Cations. Iron III Sulfate Formula.
Iron III Sulfate formula or the ferric sulfate is an inorganic salt with the formula Fe 2 SO 4 3. It is yellow in colour and is soluble in water. The molecule is formed of Fe 3 cation and SO 42 anion.
It is found in a wide variety of minerals but mainly it is found in marcasite and pyrite.