
Get your answers by asking now. Get your answers by asking now.
That is they have a net charge of 0.
Formula for sulfite ion. Sulfite is a sulfur oxoanion that is the conjugate base of hydrogen sulfite H2SO3. It is a sulfur oxoanion a sulfur oxide and a divalent inorganic anion. It is a conjugate base of a hydrogensulfite.
Sulfite ion is readily oxidized to sulfate. On prolonged exposure to air this oxidation occurs with atmospheric oxygen. 4 2 SO 3 2 aq O 2 g 2 SO 4 2 aq Sulfite or sulfur dioxide will decolorize permanganate.
What is the formula for sulfite ion. Get your answers by asking now. Ask Question 100.
Join Yahoo Answers and get 100 points today. To write the formula for Sodium sulfite well use the Periodic Table a Common Ion. In this video well write the correct formula for Sodium sulfite Na2SO3.
HSO3-Hydrogen sulfite1- bisulfite ion. Zn2 and OH- would form Zn OH2 thats 1 Zns and 2 OH Ag and CrO42- form Ag2 CrO4 Ba2 and SO32- form BaSO3 Rb and SO42- form Rb2 SO4. Sulfite Ion Formula Sulfite ion also written as sulphite is an ion that is present in many binaries salts largely used in chemical industries.
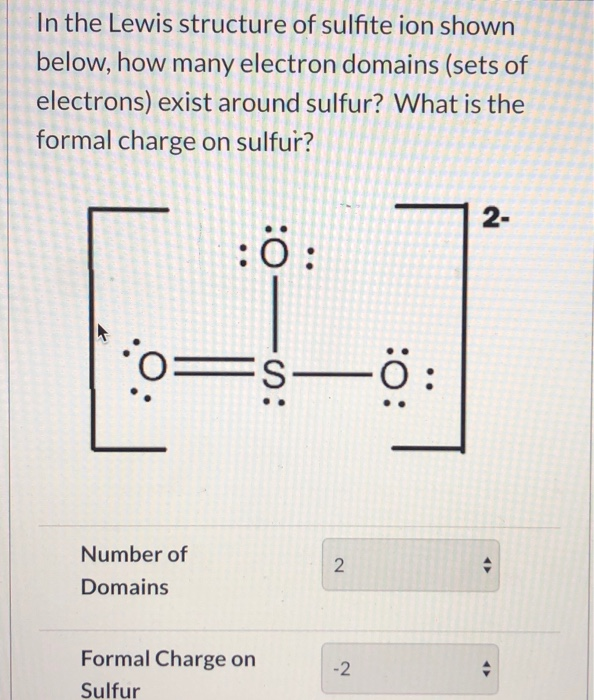
The sulphite ion chemical formula is SO 3 2-. Total valence electrons pairs. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells.
Total electron pairs are determined by dividing the number total valence electrons by two. For SO 32- ion Total pairs of electrons are 13. The sulfite ion is SOx2 3.
Ionic compounds are neutral. That is they have a net charge of 0. We need 2 N a ions to neutralize the charge of SOx2 3.
So moving forward with this knowledge the formula for the ionic compound is. SO 3 2-sulfite ion. SO 3 2-sulfite ion.
The formula for sulfite ion is SO3-2. The ion sulfite has the chemical formula SO2-3. The hydrogen sulfite ion has the chemical formula HSO-3.
The formula for the sulfite ion is mathrmSO_32-. What is the formula for sulfuric acid. Sodium sulfide another ionic compound has the formula Na 2 S.
This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to find the correct ratio of ions so that you can write a correct formula. The chemical formula for hydrogen sulfite is HSO3-.
An ion of hydrogen sulfite is made up of one atom of hydrogen represented by H three atoms of oxygen represented by O3 and one atom of sulfur represented by S. The ion has a net ionic charge of negative one. The formula for sodium sulfide is N a2S.
Since this is an ionic compound you must balance the charges so that overall charge of the compound is neutral. Sodium an alkali metal has a tendency to lose one electron. As a result sodium normally carries a positive one charge.