Draw the electron dot structure. Lewis dot diagram for sulfur.

Learn vocabulary terms and more with flashcards games and other study tools.
Electron dot structure for sulfur. Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4. Sulfur Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Electron dot diagram for sulfur.
The electron dot diagram for a lone uncharged sulfur particle is an s with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1. A oxygen 2 ion b sulfur 2 ion c antimony d aluminum. In order to write the sulfur electron configuration we first need to know the number of electrons for.
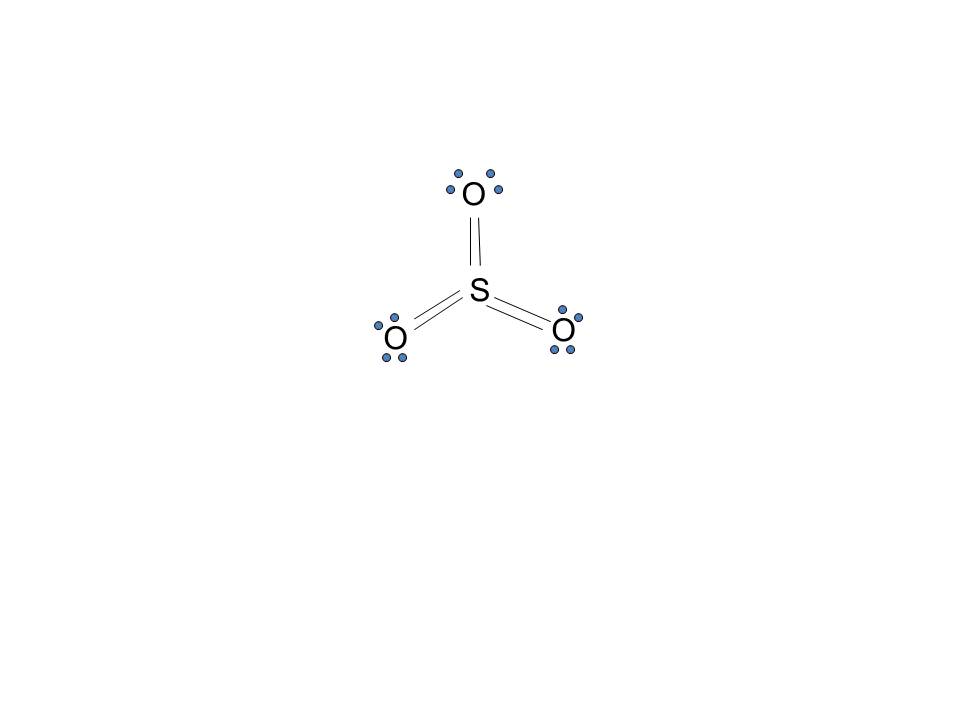
A neutral sulfur atom has 6 electrons regardless of the isotope. Lewis dot diagram for sulfur. To create the lewis structure of so2 you need to arrange the eight valence electrons on the sulphur.
To design the best lewis structure you also need to calculate the formal charge of every atom too. A neutral sulfur atom has 6 electrons regardless of the isotope. Lewis dot diagram for sulfur.
The key is to understand the steps and practice. The lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur s. Using electron-dot diagrams which show only the outermost shell electrons show how a molecule of nitrogen N2 is formed from two nitrogen atoms.
Asked Aug 14 2019 in Class X Science by navnit40. Draw the electron dot stucture of a molecule of sulphur which is made up of eight atoms of sulphur. Since an electron dot structure surrounds an elemental symbol with one dot for every valence electron that the element contains sulfurs elemental symbol must be surrounded by 6 dots.
Based on the rules given above the dot representing sulfurs first valence electron can be placed on any side of the symbol as shown below in Figure PageIndex1. What is the electron configuration for Sulfur and Chlorine. As we know sulfur contain 16 electrons around the atom and Chlorine contain 17 electrons so put these electrons in orbitals according to the sequence.
Hence sulfur electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4 And chlorine electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 5. Electron Dot Structure For Sulfur. False that would be a ionic bonds.
Covalent bonds share electrons. The stronger the bond the higher the boiling and melting point. Covalent bonds are weak compared to Ionic or hydrogen bonds.
Draw a valid electron dot structure for each of the given elements. Since fluorine is found in Group 7A of the periodic table it contains 7 valence electrons. Sulfur which is located in Group 6A has 6 valence electrons.
A chemically-correct electron dot structure for each of these elements is shown below. Sulfur is in Group 16 sometimes called Group VI or 6A. Since it is in Group 6 it will have 6 valence electrons.
When you draw the Lewis structure for Sulfur youll put six dots or valance electrons around the element symbol S. Click to see full answer. When You Draw An Electron-dot Structure For Sulfur Trioxide SO3 The Bonds Between Sulfur And Oxygen Are A All Double BondsB Two Single Bonds And One Double BondC All Single BondsD One Single Bond And Two Double Bonds.
This problem has been solved. Click hereto get an answer to your question 20. Write the electron - dot structure for calcium and sulphur.
B Show the formation of Cas by the transfer of electrons. Draw the electron dot structure of the polyatomic boron tetrafluoride anion BF 4-. Draw the electron dot structures for sulfate SO 4 2- and carbonate CO 3 2-.
Sulfur and carbon are the central atoms respectively. Draw the electron dot structure. Start studying Electron Dot Diagrams and Lewis Structures.
Learn vocabulary terms and more with flashcards games and other study tools. Log in Sign up. Upgrade to remove ads.
Electron Dot Diagrams and Lewis Structures. Electron dot diagram for Sulfur.