The chemical symbol for Yttrium is Y. Furthermore it forms several compounds such as oxalate hydroxide and fluoride that are insoluble in water.

Electron Configuration and Oxidation States of Yttrium.
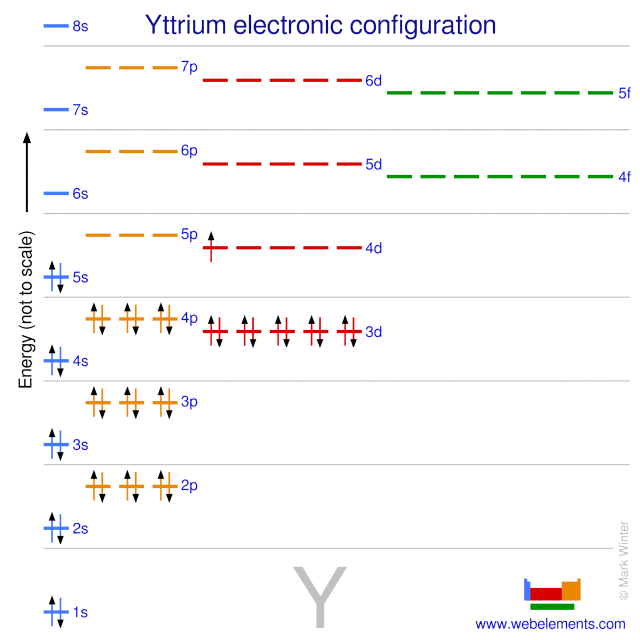
Electron configuration of yttrium. Full electron configuration of yttrium. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d1 5s2. Yttrium is a chemical element with atomic number 39 which means there are 39 protons and 39 electrons in the atomic structure.
The chemical symbol for Yttrium is Y. Electron Configuration and Oxidation States of Yttrium. Electron configuration of Yttrium is Kr 4d1 5s2.
Possible oxidation states are 3. Generally yttrium electronic configuration is Kr4d¹5s². Yttrium commonly has an oxidation state of 3 since it gives three valence electrons.
Furthermore it forms several compounds such as oxalate hydroxide and fluoride that are insoluble in water. And compounds such as chloride sulfate bromide etc. Have high solubility in water.
7 rows Electron configuration Kr 4d 1 5s 2 CAS number. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 1 5s 2 Back to key information about the elementBack to key information about the element. Yttrium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s2 4 d1 Abbreviated Electron Configuration Kr 4d1 5s2 Sources Found in minerals such as monazite xenotime and yttria.
Atomic Symbol Y Uses Combined with europium to make red phosphors for color TVs. Yttrium oxide and iron oxide combine to form a crystal garnet used in radar. 13 rows The number of electrons in each elements electron shells particularly the outermost valence.
No because 4d1 is actually a higher energy level. If you look on the periodic table assuming you know how to do this youll see that. 119 rows Its electron configuration is.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Yttrium atoms have 39 electrons and the shell structure is 281892. The ground state electron configuration of ground state gaseous neutral yttrium is Kr.
5s 2 and the term symbol is 2 D 32. Ground state Electron Configuration. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 1 5s 2.
447 gcm 3 300 K Atomic Volume. 1980 cm 3 mol 300 K Number of stable Isotopes. 1 89 Y Note.
19 other unstable isotopes have been identified. Atomic Structure of Yttrium. 198cm 3 mol.
Cross Section Thermal Neutron Capture σ a barns. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6. The electron configuration and orbital diagram of helium are.
The n 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as.
The ground state electronic configuration of Neutral Yttrium atom is Kr 4d1 5s2. The portion of Yttrium configuration that is equivalent to the noble gas. Yttrium atoms have 39 electrons and the shell structure is 28.
The ground state electron configuration of ground state gaseous neutral yttrium is Kr. Write the ground state electron configuration of yttrium in both the long format and the short nobel gas format. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 1 Kr5s 2 4d 1.