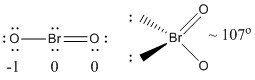
This is an addendum to a previous video where the formal charges where not taken into account in the Lewis structure. Dioxidobromine BrO2 CID 5460629 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
Bromoether Br2O CID 14513628 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
Bro2 lewis structure. Subtract step 1 total from step 2 24-186e- Step 4. Find number of bonds by diving the number in step 3 by 2 because each bond is made of 2 e- 6e-2 3 bond pairs. Find the number of nonbonding lone pairs e-.
Subtract step 3 number from step 1. 18-6 12e-6 lone pairs. Dioxidobromine BrO2 CID 5460629 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
Lewis Structure also gives an insight into the electron configuration of an atom and the way in which it can aim to achieve stability through equilibrium. While the Lewis Structure provides an idea about the physical attributes of the compound its representation is limited since it is a 2-dimensional model. It also does not reflect upon the molecular design geometry or the 3-dimensional.
Bromous acid HBrO2 or BrHO2 CID 165616 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. BrO Lewis Structure - YouTube. Get YouTube without the ads.
Bromoether Br2O CID 14513628 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. The BrO 2-Lewis structure has a total of 20 valence electrons. This includes the electron represented by the negative charge in BrO 2 -.
You need to put brackets around the BrO 2 - Lewis structure as well as a negative charge to show that the structure is a negative ion. Lewis structure is also known as Kekule structure in which the distribution of valence electrons is shown. It is used to show how the electrons are arranged around an individual.
Resonance structures are used when it is difficult to write Lewis diagrams but. Bromine in bromite ion has an expanded octet and is bonded to two oxygen atoms. Each bond is identical and has a bond order of 15.
There is no need for resonance structures if we fudge the Lewis diagram to show two identical bonds with bond orders of 15. Below that are the two resonance structures which when averaged give the top diagram. A step-by-step explanation of how to draw the BeBr2 Lewis Dot StructureFor the BeBr2 structure use the periodic table to find the total number of valence el.
This is an addendum to a previous video where the formal charges where not taken into account in the Lewis structure. As a result the answer in the previou. Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO 2.
It forms unstable yellow It is similar to chlorine dioxide the dioxide of its halogen neighbor one period higher on the periodic table. Read full article at Wikipedia. Edit article at Wikipedia.
A step-by-step explanation of how to draw the NO Lewis Dot Structure Nitronium ionFor the NO structure use the periodic table to find the total number o.
