Chemical Elements Periodic Table. Al 2 SO 4 3 18H 2 O.
Tristrioxosulfato IV de dialuminio.
Al2so33. When in water Al2SO33 will hydrolysis with products AlOH3 and SO2. Chemical Elements Periodic Table. Compound Name Formula Search.
Moles to Grams Calculator. Elemental composition of Al2SO33. Element Symbol Atomic weight Atoms Mass percent.
Formula in Hill system is Al2O9S3. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and. Explanation of how to find the molar mass of Al2SO33.
Aluminum SulfiteA few things to consider when finding the molar mass for Al2SO33- make sure you h. Did you mean Al2SO43. Molare Masse of Al2SO33 is 2941527 gmol Berechnen Sie das Gewicht von Al2SO33 oder Mol.
In this video well balance the equation Al2SO33 NaOH Na2SO3 AlOH3 and provide the correct coefficients for each compoundTo balance Al2SO33 Na. Friendo 17 August 0829. AnswerIn the chemical formula Al2 SO4 3 the Al2 means there two aluminium atoms or ions.
The SO4 is a sulfate ion and SO4 3 means there are 3 sulfate ions. The number 3 before Al2 SO4 3 means there are three times the number of atoms and ions of the chemical formula. Thus Al2SO33 is the limiting reagent.
2 The maximum number of moles of product you can get is if all the limiting reagent reacts fully. In this case moles of product will be that formed from. Moles Al2 SO33 3894 29415 132.
Moles Na2SO3 132 x 3 396. Mass Na2SO3 396 x 126 gmol 49896 g. Al2 SO33—–siarczan IV glinu.
Al 2 SO 3 3. Tristrioxosulfato IV de dialuminio. To find the total number of atoms in Al2CO33 Aluminum carbonate well add up the number of each type of atom.
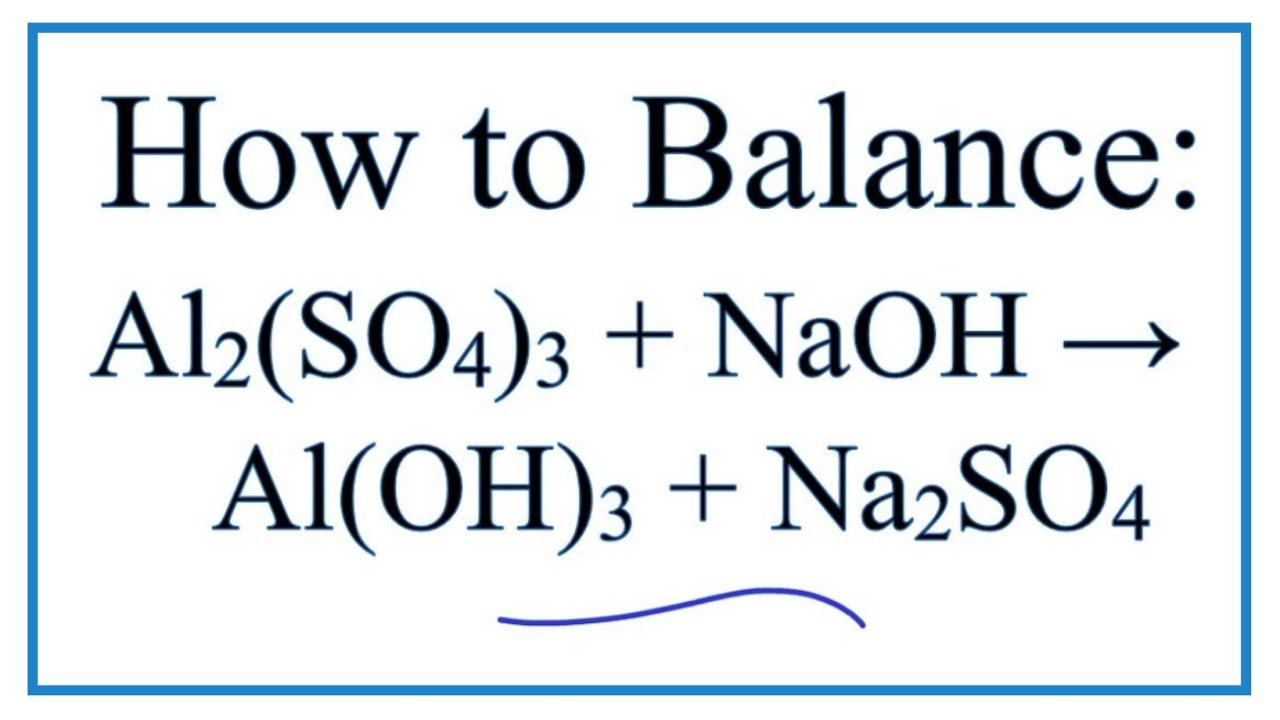
The small number after the element symbol i. The small number after the. In this video well balance the equation Al2SO43 NaOH AlOH3 Na2SO4 and provide the correct coefficients for each compoundTo balance Al2SO43 Na.
You need to find percent composition. To do that you need to get the molar masss of all the elements from the periodic table. Al 2698 x 2 5396g.
S 32065 x 3 96195g. O 159943 x 12. Anhydrous aluminum sulfate is a white crystalline solid.
Aluminum sulfate is also obtained as an 18-hydrate Al2SO4318H2O. Both forms are soluble in water noncombustible and nontoxic. The primary hazard is the threat to the environment.
Um die Oxidationszahlen Elemente in der chemischen Verbindung zu berechnen geben Sie deren Formel ein und klicken Sie auf Berechnen zum Beispiel. Ca2 HF2- Fe4 Fe CN63 NH4NO3 so42- ch3cooh cuso45h2o. Die Oxidationszahl Oxidationsstufe des Atoms ist die Ladung dieses Atoms nach ionischer Annährung seiner.
Al2SO33 NaOH Na2SO3 AlOH Enter a chemical equation to balance. Al2So33 might be an improperly capitalized. Al 2 SO 4 3 18H 2 O.